Computer Software Validation Services
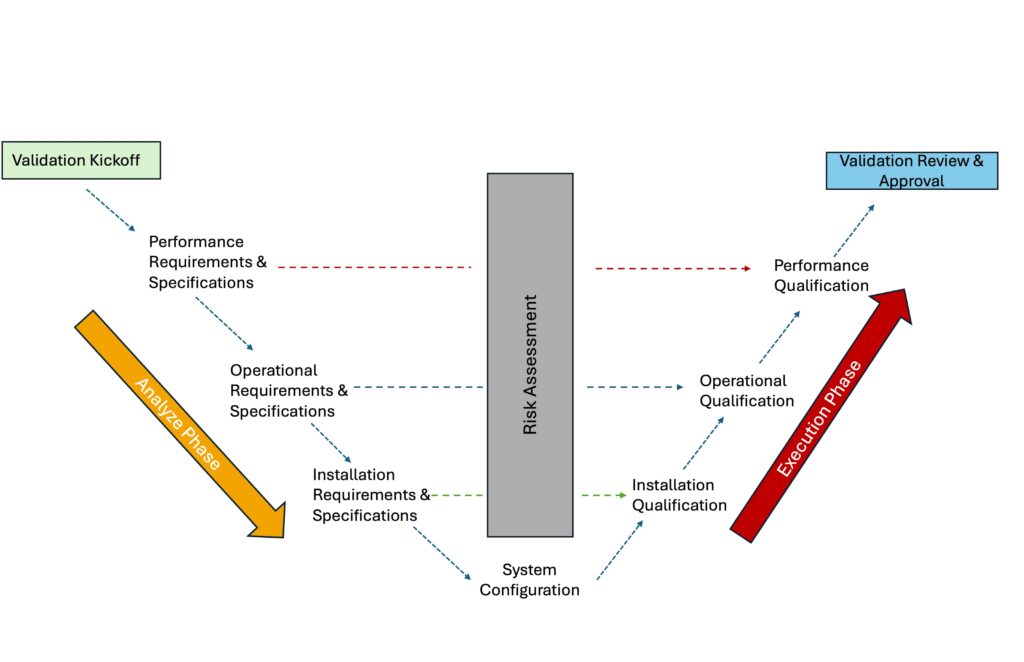
Validation Process Overview
Our process is divided into two phases. The diagram outlines a risk-based Computer System Validation (CSV) process, which consists of two phases: Analysis and Execution.
Analyze Phase: Begins with a Validation Kickoff, where system requirements are defined within the following:
- Performance Requirements
- Operational Requirements
- Installation Requirements
Execution Phase: The system is configured and validated in three stages
- Installation Qualification (IQ): Confirms correct setup.
- Operational Qualification (OQ): Verifies functional performance.
- Performance Qualification (PQ): Confirms the system performs as intended under real-use conditions.

We recommend a risk-based approach
Instead of validating everything equally, we focus on what matters most: functions and components that impact patient safety, product quality, or regulatory compliance.
In short, we don’t overvalidate. We validate what counts.

Why Our Validation Approach Works
We don’t just validate systems—we teach your team why it matters, and how to do it smarter.
Understanding CSV Basics
CSV (Computer System Validation) is the traditional FDA-compliant validation method.
It requires detailed documentation to prove that a computerized system consistently works as intended.
Heavily regulated under FDA 21 CFR Part 11 for electronic records and signatures.
The main goal: Document every step of the validation process, not just the testing itself.
Getting Started
Access the System
Log in with your assigned credentials.
Ensure your role and permissions match your function.
Familiarize with CSV’s Core Documentation
User Requirement Specification (URS)
Functional Requirement Specification (FRS)
Design Specification (DS)
Test Plans and Protocols
Validation Summary Reports
Recording and Managing Work
Material Management:
Log all material entries with full traceability.
Keep all labels, scans, and receipts documented.
Production Recording:
Complete the electronic batch record (eBR) with full details for each step.
Include time stamps, operator names, and electronic signatures.
Quality Control:
Record every QC inspection in detail.
Maintain an audit trail for any corrections or changes.
Risk and Validation Process
Plan the Validation
Define scope, objectives, and compliance requirements.
List all system functions to be tested.
Write Test Protocols
Create step-by-step scripts for each test.
Include expected results and acceptance criteria.
Execute and Record Tests
Document every test run, whether it passes or fails.
Capture screenshots, logs, and evidence.
Review and Approve
Supervisors and QA must sign off each test and protocol.
Compliance Tracking
Documentation is king in CSV:
Maintain SOPs, test scripts, and results in the validation binder or system repository.
Ensure all Part 11 compliance steps (electronic signatures, audit trails) are followed.
Change Control:
Any change to the system triggers a re-validation cycle.
Validation Deliverables
You will be able to generate and access:
Validation Master Plan (VMP)
URS, FRS, DS Documents
Test Protocols and Reports
Validation Summary Report
Evidence of Testing Archive
Ongoing Use
Schedule periodic revalidation for regulated systems.
Keep all records ready for FDA audit.
Train new users in CSV protocols before they operate the system.
Get started with your personalized demo.
See how it could work for you- book a demo with one of our experts and we’ll show you what the quality operations management platform can do.
Here’s what to do
+ Fill in your details and click “Submit”
+ We’ll get in touch to book a date and time
for a demo.
+ On the day, we’ll walk you through the platform
and show you how it all comes together.